Early findings from Europa Uomo’s study
Early findings from the first prostate cancer quality of life study conducted by patients themselves show that significant numbers of men treated for the disease are struggling with mental health, sexual and tiredness problems after treatment.
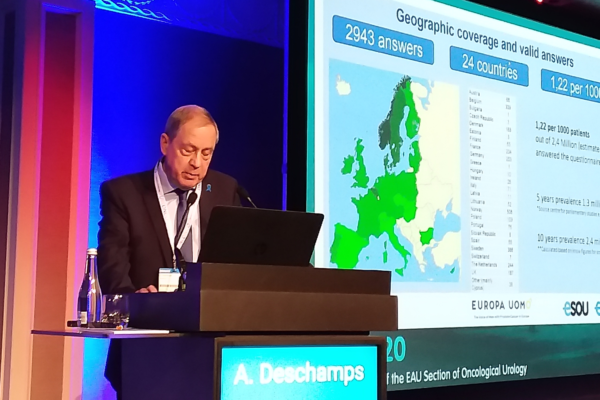
The Europa Uomo Patient Reported Outcomes Study (EUPROMS) is using data from 2,943 questionnaire responses from men who have had prostate cancer from 24 European countries. The preliminary findings have been announced by Europa Uomo Chairman André Deschamps at the European Association of Urology’s oncology meeting in Dublin (pictured).
Around 45% of men reported experiencing anxiety or depression after treatment. And an analysis of survey responses using the EPIC-CP quality of life instrument indicated that lack of sexual function is having the highest impact on quality of life, with a quality of life score significantly lower that recorded in previous clinical studies. Use of, and satisfaction with, medication and devices to help erections is very low.
The survey returns also reveal that patients who have received two or more treatments have significantly lower quality of life scores.
“Our questionnaire yielded an impressive number of responses, representing a good cross-section of men of different ages and different stages of treatment,” said André Deschamps. “We have a good picture of prostate cancer survivors across Europe.”
“We conducted the study because we wanted to give patients an idea of what to expect after treatment. We also wanted to investigate the impact of diagnosis on quality of life and to raise awareness of quality of life – not just survival – as an important issue in prostate cancer.”
The announcement of these early findings precedes a full analysis of results, led by Professor Monique Roobol of the Erasmus University Medical Centre, Department of Urology, Rotterdam. A detailed analysis is expected to be presented in March at the European Association of Urology’s conference in Amsterdam.